Abstract
Introduction: Mezigdomide (MEZI), a novel oral CELMoD® agent with enhanced tumoricidal and immune-stimulatory effects compared to immunomodulatory drugs (IMiDs®), induces maximal degradation of Ikaros and Aiolos, leading to increased apoptosis in myeloma cells. Preclinically, MEZI demonstrated potent synergy with DEX, proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (mAbs). CC-92480-MM-001 (NCT03374085) is an ongoing phase 1/2 trial evaluating MEZI alone or in combination with DEX in pts with RRMM; in phase 1, the recommended phase 2 dose (RP2D) of MEZI in combination with DEX was selected at 1 mg once daily for 21/28 days (Richardson PG, et al. J Clin Oncol 2020;30[15 suppl]:8500). Here we report results from the dose-expansion cohort of MEZI + DEX in pts with heavily pretreated RRMM.
Methods: Eligible pts had: RRMM; ≥ 3 prior lines of therapy; disease progression on or within 60 days of last myeloma therapy; refractoriness to LEN/POM, a PI, a glucocorticoid, and an anti-CD38 mAb. MEZI 1 mg was given orally on days 1-21 of each 28-day cycle, plus weekly DEX (40 mg; 20 mg if > 75 years of age). Primary objective was to determine efficacy by overall response rate (ORR); secondary objectives included safety/tolerability and additional efficacy assessments. Efficacy was also assessed in pts with plasmacytomas and in pts with prior anti-B-cell maturation antigen (BCMA) therapy. Pharmacodynamics was an exploratory objective.
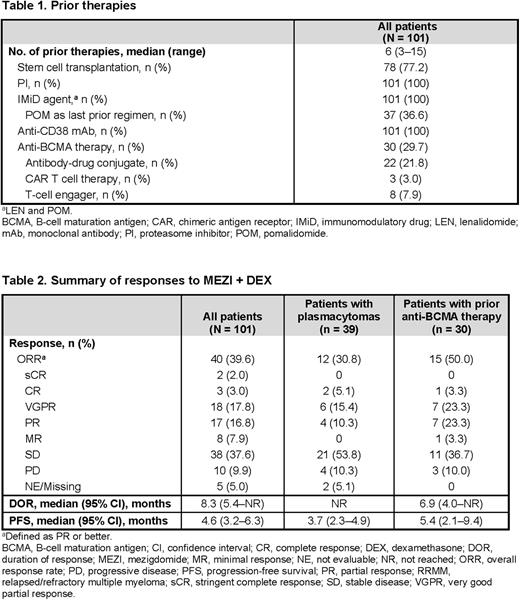
Results: As of May 24, 2022, 101 pts had received MEZI + DEX at the RP2D. Median age was 67 (range 42-85) years, median time since initial diagnosis was 7.4 (1.1-37.0) years, and 20.8% of pts had ISS stage III at study entry. Plasmacytomas were present in 38.6% of pts and 36/101 pts had high-risk cytogenetics (55/101 pts were not evaluable). Median number of prior regimens was 6 (range 3-15). Prior therapies included stem cell transplantation (77.2%) and anti-BCMA therapy (29.7%) (Table 1). All pts were refractory to last myeloma regimen and triple-class refractory (refractory to an IMiD agent [LEN/POM], a PI, and an anti-CD38 mAb), and 39.6% of pts were refractory to LEN, POM, ≥ 2 PIs, and an anti-CD38 mAb. Median follow-up was 5.8 (range 0.5-19.0) months, with a median number of 4 (1-18) cycles received and 23 (22.8%) pts continued on treatment. The main reason for discontinuation was progressive disease (50.5%).
ORR was 39.6%, with 2 (2.0%) stringent complete responses, 3 (3.0%) complete responses, 18 (17.8%) very good partial responses, and 17 (16.8%) partial responses (Table 2). While data are not yet mature, the preliminary median duration of response was 8.3 (95% confidence interval [CI] 5.4-not reached) months and median progression-free survival was 4.6 (95% CI 3.2-6.3) months. ORR was 30.8% in pts with plasmacytomas (N = 39) and 50.0% in pts with prior anti-BCMA therapy (N = 30).
Grade (Gr) 3/4 treatment-emergent adverse events (TEAEs) were reported in 90 (89.1%) pts. Most frequent (≥ 20% pts) hematologic Gr 3/4 TEAEs were neutropenia (74.3%, with 14.9% febrile neutropenia), anemia (32.7%), and thrombocytopenia (25.7%). Gr 3/4 infections were observed in 32.7% of pts; Gr 3/4 pneumonia and COVID-19 were present in 9.9% and 5.0% of pts, respectively. The occurrence of other Gr 3/4 non-hematologic TEAEs was generally low, including gastrointestinal disorders (5.9%), fatigue (4.0%), and rash (1.0%). Seventy-two (71.3%) pts and 29 (28.7%) had MEZI dose interruptions and reductions due to TEAEs, respectively; 6 (5.9%) pts discontinued MEZI due to TEAEs, with 1 pt due to hematological TEAEs.
MEZI induced potent pharmacodynamic effects in this cohort, including substrate degradation and increases in activated and proliferating T cells in all pts, as well as in pts directly refractory to POM-based therapies demonstrating the mechanism of MEZI action is still functioning in this heavily pretreated population.
Conclusions: MEZI + DEX had a manageable safety profile and demonstrated promising efficacy in pts with triple-class refractory RRMM, including pts with prior BCMA-targeted therapies, with an ORR of 40% and 50% respectively. These results support the development of MEZI in pts with MM. MEZI is currently being evaluated in combination with standard therapies in MM as part of a large, ongoing phase 1/2 trial (NCT03989414) and phase 3 trials in combination with PIs are planned.
Disclosures
Richardson:Regeneron: Consultancy; AstraZeneca: Consultancy; Protocol Intelligence: Consultancy; Secura Bio: Consultancy; GlaxoSmithKline: Consultancy; Oncopeptides: Consultancy, Research Funding; Sanofi: Consultancy; Karyopharm: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Takeda, Abbvie, GSK, and Celgene: Consultancy; Takeda, Celgene, and GSK: Honoraria; Takeda: Research Funding; Takeda and GSK: Other: Travel expenses from Takeda and GSK. Trudel:Janssen: Honoraria, Research Funding; AstraZeneca: Honoraria; Karyopharm: Honoraria; Takeda: Honoraria; Forus: Consultancy; Sanofi: Honoraria; Genentech: Research Funding; Pfizer: Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; GlaxoSmithKline: Consultancy, Honoraria, Research Funding. Quach:AbbVie: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding; Karyopharm: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Antengene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role , Research Funding; CSL: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees, Other: receipt of free drug for investigator-initiated study, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: leadership or fiduciary role, receipt of free drug for investigator-initiated study , Research Funding. Popat:Takeda: Research Funding; Janssen, Takeda, Celgene, and GSK: Honoraria; Janssen, Takeda, GSK: Other: Travel expenses from Janssen, Takeda, GSK; Takeda, AbbVie, GlaxoSmithKline, and Celgene: Consultancy; BMS: Honoraria; Roche: Honoraria; Janssen: Honoraria; GSK: Honoraria, Research Funding. Lonial:Celgene, Janssen, Takeda: Research Funding; Novartis, BMS, GSK, Amgen, Merck, Janssen: Honoraria; AbbVie, Bluebird, Bristol-Myers Squibb, Celgene, GlaxoSmithKline, Janssen, Novartis Pharma, and Takeda.: Consultancy. Orlowski:CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding; Asylia Therapeutics, Inc.: Current equity holder in private company. Kim:Janssen: Consultancy, Research Funding; LG Chem: Membership on an entity's Board of Directors or advisory committees; BMS: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Mateos:Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides: Membership on an entity's Board of Directors or advisory committees; Janssen, Celgene, Takeda, Amgen, GSK, AbbVie, Pfizer, Regeneron, Roche, Sanofi, Oncopeptides, Seagen: Honoraria. Pawlyn:Abbvie: Consultancy; Sanofi: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Travel support; Celgene/BMS: Consultancy, Honoraria. Ramasamy:Takeda, Bristol Myers Squibb, Janssen, Amgen, GlaxoSmithKline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding, Speakers Bureau; Sanofi, Adaptive biotech, Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Martinez-Lopez:Bristol Myers Squibb: Consultancy, Honoraria, Other: Support for attending meetings and/or travelIncyte; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Novartis: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Roche: Consultancy, Honoraria, Other: Support for attending meetings and/or travel ; Sanofi: Consultancy, Honoraria, Other: Support for attending meetings and/or travel . Spirli:Bristol Myers Squibb: Current Employment. Gong:BMS: Current Employment. Amatangelo:Abbvie: Current equity holder in publicly-traded company; Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Katz:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Maciag:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Peluso:Bristol Myers Squibb: Current Employment, Current equity holder in publicly-traded company. Bahlis:AbbVie, Amgen, Bristol Myers Squibb, Celgene, Forus, Janssen, Genentech, GSK, Karyopharm, Novartis, Pfizer, Takeda, Sanofi: Consultancy; Pfizer: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal